Mannitol Salt Agar Agar : Composition,
Principle and Use
Intended Use:
Recommended for the isolation of pathogenic Staphylococci from clinical and
non-clinical samples
Composition
Ingredients
|
Gms / Litre
|
Proteose Peptone
|
10.00
|
HM peptone B
or Beef extract
|
1.0
|
Sodium
chloride
|
75.00
|
D-Mannitol
|
10.00
|
Phenol red
|
0.025
|
Agar
|
15.00
|
pH after
sterilization ( at 25°C) 7.4±0.2
|
Directions:
Suspend 111.02 grams in 1000 ml purified / distilled water. Heat to boiling
to dissolve the medium completely. Sterilize by autoclaving at 15 lbs pressure
(121°C) for 15 minutes. Cool to 45-50°C.
If desired, add 5% v/v Egg Yolk Emulsion. Mix well and pour into
sterile Petri plates.
Note : This product contains 7.5% Sodium chloride as one of its
ingredients. On repeated exposure to air and absorption moisture sodium
chloride has tendency to form lumps, therefore it is strongly recommend storage
in tightly closed containers in dry place away from bright light.
Principle And Interpretation:
Staphylococci are widespread in nature, although they are mainly found on
the skin, skin glands and mucous membranes of mammals and birds.The
coagulase-positive species i.e Staphylococcus aureus is well documented as a
human opportunistic pathogen.
The ability to clot plasma continues to be the most widely used and
accepted criterion for the identification of pathogenic staphylococci associated
with acute infections.
Staphylococci have the unique ability of growing on a high salt containing
media.
Isolation of coagulase-positive staphylococci on Phenol Red Mannitol Agar
supplemented with 7.5% NaCl was studied by Chapman.
The resulting Mannitol Salt Agar Base is recommended for the isolation of
coagulase-positive staphylococci from cosmetics, milk, food and other specimens.
The additional property of lipase activity of Staphylococcus aureus can be
detected by the addition of the Egg Yolk Emulsion.
The lipase activity can be visualized as yellow opaque zones around the colonies.
HM peptone B and proteose peptone supply essential growth factors and trace
nutrients to the growing bacteria.
Sodium chloride serves as an inhibitory agent against bacteria other than
staphylococci.
Mannitol is the fermentable carbohydrate, fermentation of which leads to
acid production, detected by phenol red indicator.
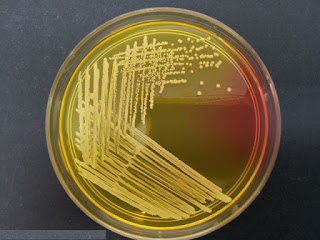
S.aureus ferment mannitol and produce yellow coloured colonies surrounded
by yellow zones.
Coagulase-negative strains of S.aureus are usually mannitol non-fermenters
and therefore produce pink to red colonies surrounded by red-purple zones.
Presumptive coagulase-positive yellow colonies of S. aureus should be
confirmed by performing the coagulase test [tube or slide]. Lipase activity of
S.aureus can be detected by supplementing the medium with egg yolk emulsion.
A possible S.aureus must be confirmed by the coagulase test.
Also the organism should be subcultured to a less inhibitory medium not
containing excess salt to avoid the possible interference of salt with
coagulase testing or other diagnostic tests (e.g. Nutrient Broth). Few strains
of S.aureus may exhibit delayed mannitol fermentation.
Limitations
1. A possible S.aureus must be confirmed by the coagulase test.
2. The organism should be subcultured to a less inhibitory medium not
containing excess salt to avoid the possible interference of salt with
coagulase testing or other diagnostic tests (e.g. Nutrient Broth). 3. Few strains of S.aureus may exhibit delayed mannitol fermentation.
Negative results should therefore be re-incubated for an additional 24 hours
before being discarded.

Emoticon Emoticon