The activity or potency of antibiotics may be
demonstrated under suitable conditions by their inhibitory effect on
microorganisms. Antimicrobial activity can be checked by microbial method and
chemical method also.
Two general methods are employed, the zone of inhibition
or “plate” assay and the
turbidimetric or “tube” assay.
The first depends upon diffusion of the antibiotic from a
vertical cylinder through a solidified agar layer in a petri dish or plate to
an extent such that growth of the added microorganism is prevented entirely in
a circular area or “zone” around the
bore containing a solution of the antibiotic.
The turbidimetric method depends upon the inhibition of
growth of a microbial culture in a uniform solution of the antibiotic in a
liquid medium that promote its rapid growth in the absence of the antibiotic.
Requirements:All equipment is to be thoroughly cleaned before and
after each use.Glassware for holding and transferring test organisms is
sterilized by dry heat or by steam.
Temperature Control is required in several stages of a microbial
assay, when culturing a microorganism and preparing its inoculum, and during
incubation in plate and tube assays. Maintain the temperature of assay plates
at ±0.5°C of the
temperature selected by circulated air or water.
Spectrophotometer is required in turbidimetric
method. Measuring transmittance within a
fairly narrow frequency of wavelength of the light source can be used i.e
580-nm filter or a 530-nm filter for reading the absorbance in a tube assay.
Set the instrument at zero absorbance with clear,
uninoculated broth prepared as specified for the particularantibiotic, including the same amount of test solution as
found in each sample.NOTE—Either absorbance or transmittance
measurement may be used for preparing inoculum.
Cylinder-Plate Assay
For assay plates, use glass or plastic petri dishes (approximately 20 × 100 mm) having covers of suitable material. For assay cylinders, use stainless steel or porcelain cylinders with the following dimensions, each dimension having a tolerance of ±0.1 mm: outside diameter 8 mm; inside diameter 6 mm; and length 10 mm. Clean cylinders to remove all residues. Wash with acid, e.g. with about 2 N nitric acid or with chromic acid if required.
Turbidimetric Assay
For assay tubes, use glass or plastic test tubes, e.g.,
16 × 125 mm or 18 × 150 mm that
are relatively uniform in length, diameter, and thickness and substantially
free from surface blemishes and scratches. Tubes that are to be placed in the
spectrophotometer are matched and are without scratches or blemishes. Clean
thoroughly to remove all antibiotic residues and traces of cleaning solution,
and sterilize tubes that have been used previously, before subsequent use.
MEDIA AND DILUENTS
Media
The media required for the preparation of test organism inoculum
are made from the ingredients listed below.
Dissolve the ingredients in water to make 1 L, and adjust
the pH with either 1 N sodium hydroxide or 1 N hydrochloric acid as required,
so that after steam sterilization the pH is as specified.
MEDIUM 1:
|
|
Peptone
|
6.0 g
|
Pancreatic Digest of Casein
|
4.0 g
|
Yeast Extract
|
3.0 g
|
Beef Extract
|
1.5 g
|
Dextrose
|
1.0 g
|
Agar
|
15.0 g
|
Water
|
1000 ml
|
pH after sterilization: 6.6 ± 0.1.
|
|
MEDIUM 2:
|
|
Peptone
|
6.0 g
|
Yeast Extract
|
3.0 g
|
Beef Extract
|
1.5 g
|
Agar
|
15.0 g
|
Water
|
1000 ml
|
pH after sterilization: 6.6 ± 0.1.
|
|
MEDIUM 3:
|
|
Peptone
|
5.0 g
|
Yeast Extract
|
1.5 g
|
Beef Extract
|
1.5 g
|
Sodium Chloride
|
3.5 g
|
Dextrose
|
1.0 g
|
Dibasic Potassium Phosphate
|
3.68 g
|
Monobasic Potassium Phosphate
|
1.32 g
|
Water
|
1000 ml
|
pH after sterilization: 7.0 ± 0.05.
|
|
MEDIUM 4:
|
|
Dextrose
|
1.0 g
|
Peptone
|
6.0 g
|
Yeast Extract
|
3.0 g
|
Beef Extract
|
1.5 g
|
Agar
|
15.0 g
|
Water
|
1000 ml
|
pH after sterilization: 6.6 ± 0.1.
|
|
MEDIUM 5:
|
|
Peptone
|
6.0 g
|
Yeast Extract
|
3.0 g
|
Beef Extract
|
1.5 g
|
Agar
|
15.0 g
|
Water
|
1000 ml
|
pH after sterilization: 7.9 ± 0.1.
|
|
MEDIUM 8:
|
|
Peptone
|
6.0 g
|
Yeast Extract
|
3.0 g
|
Beef Extract
|
1.5 g
|
Agar
|
15.0 g
|
Water
|
1000 ml
|
pH after sterilization: 5.9 ± 0.1.
|
|
MEDIUM 9:
|
|
Pancreatic Digest of Casein
|
17.0 g
|
Papaic Digest of Soybean
|
3.0 g
|
Sodium Chloride
|
5.0 g
|
Dibasic Potassium Phosphate
|
2.5 g
|
Dextrose
|
2.5 g
|
Agar
|
20.0 g
|
Water
|
1000 ml
|
pH after sterilization: 7.2 ± 0.1.
|
|
MEDIUM 10:
|
|
Pancreatic Digest of Casein
|
17.0 g
|
Papaic Digest of Soybean
|
3.0 g
|
Sodium Chloride
|
5.0 g
|
Dibasic Potassium Phosphate
|
2.5 g
|
Dextrose
|
2.5 g
|
Agar
|
12.0 g
|
Water
|
1000 ml
|
Add 10 mL of Polysorbate 80 after boiling
the medium to dissolve the agar.
|
|
pH after sterilization: 7.2 ± 0.1.
|
|
MEDIUM 11:
|
|
Peptone
|
6.0 g
|
Pancreatic Digest of Casein
|
4.0 g
|
Yeast Extract
|
3.0 g
|
Beef Extract
|
1.5 g
|
Dextrose
|
1.0 g
|
Agar
|
15.0 g
|
Water
|
1000 ml
|
pH after sterilization: 8.3 ± 0.1.
|
|
MEDIUM 13:
|
|
Dextrose
|
20.0 g
|
Peptone
|
10.0 g
|
Water
|
1000 ml
|
pH after sterilization: 5.6 ± 0.1.
|
|
MEDIUM 19:
|
|
Peptone
|
9.4 g
|
Yeast Extract
|
4.7 g
|
Beef Extract
|
2.4 g
|
Sodium Chloride
|
10.0 g
|
Dextrose
|
10.0 g
|
Agar
|
23.5 g
|
Water
|
1000 ml
|
pH after sterilization: 6.1 ± 0.1.
|
|
MEDIUM 32:
|
|
Manganese Sulfate
|
0.3 g
|
Peptone
|
6.0 g
|
Pancreatic Digest of Casein
|
4.0 g
|
Yeast Extract
|
3.0 g
|
Beef Extract
|
1.5 g
|
Dextrose
|
1.0 g
|
Agar
|
15.0 g
|
Water
|
1000 ml
|
pH after sterilization: 6.6 ± 0.1.
|
|
MEDIUM 34:
|
|
Glycerol
|
10.0 g
|
Peptone
|
10.0 g
|
Beef Extract
|
10.0 g
|
Sodium Chloride
|
3.0 g
|
Water
|
1000 ml
|
pH after sterilization: 7.0 ± 0.1.
|
|
MEDIUM 35:
|
|
Glycerol
|
10.0 g
|
Peptone
|
10.0 g
|
Beef Extract
|
10.0 g
|
Sodium Chloride
|
3.0 g
|
Water
|
1000 ml
|
Agar
|
17.0 g
|
pH after sterilization: 7.0 ± 0.1.
|
|
MEDIUM 36:
|
|
Pancreatic Digest of Casein
|
15.0 g
|
Papaic Digest of Soybean
|
5.0 g
|
Sodium Chloride
|
5.0 g
|
Agar
|
15.0 g
|
Water
|
1000 ml
|
pH after sterilization: 7.3 ± 0.1.
|
|
MEDIUM 39:
|
|
Peptone
|
5.0 g
|
Yeast Extract
|
1.5 g
|
Beef Extract
|
1.5 g
|
Sodium Chloride
|
3.5 g
|
Dextrose
|
1.0 g
|
Dibasic Potassium Phosphate
|
3.68 g
|
Monobasic Potassium Phosphate
|
1.32 g
|
Water
|
1000 ml
|
pH after sterilization: 7.9 ± 0.1.
|
|
MEDIUM 40:
|
|
Yeast Extract
|
20.0 g
|
Polypeptone
|
5.0 g
|
Dextrose
|
10.0 g
|
Monobasic Potassium Phosphate
|
2.0 g
|
Polysorbate
|
0.1 g
|
Agar
|
10.0 g
|
Water
|
1000 ml
|
pH after sterilization: 6.7 ± 0.2.
|
|
MEDIUM 41:
|
|
Pancreatic Digest of Casein
|
9.0 g
|
Dextrose
|
20.0 g
|
Yeast Extract
|
5.0 g
|
Sodium Citrate
|
10.0 g
|
Monobasic Potassium Phosphate
|
1.0 g
|
Dibasic Potassium Phosphate
|
1.0 g
|
Water
|
1000 ml
|
pH after sterilization: 6.8 ± 0.1.
|
|
Phosphate Buffers and Other SolutionsPrepare the potassium phosphate buffers required for the
antibiotic assay as below. Sterilize the buffers after preparation, and the
adjust pH specified in each case is the pH after sterilization.
BUFFER NO. 1:1% Solution, pH 6.0— Dissolve 2.0 g
of dibasic potassium phosphate and 8.0 g of monobasic potassium phosphate in
1000 mL of water. Adjust with 18 N phosphoric acid or 10 N potassium hydroxide
to a pH of 6.0 ± 0.05.
BUFFER NO. 3:0.1 M, pH 8.0— Dissolve 16.73
g of dibasic potassium phosphate and 0.523 g of monobasic potassium phosphate
in 1000 mL of water. Adjust with 18 N phosphoric acid or 10 N potassium
hydroxide to a pH of 8.0 ± 0.1.
BUFFER NO. 4:0.1 M, pH 4.5— Dissolve 13.61
g of monobasic potassium phosphate in 1000 mL of water. Adjust with 18 N
phosphoric acid or 10 N potassium hydroxide to a pH of 4.5 ± 0.05.
BUFFER NO. 6:10 PERCENT, pH 6.0— Dissolve 20.0
g of dibasic potassium phosphate and 80.0 g of monobasic potassium phosphate in
1000 mL of water. Adjust with 18 N phosphoric acid or 10 N potassium hydroxide
to a pH of 6.0 ± 0.05.
BUFFER NO. 10:0.2 M, pH 10.5— Dissolve 35.0
g of dibasic potassium phosphate in 1000 mL of water, and add 2 mL of 10 N
potassium hydroxide. Adjust with 18 N phosphoric acid or 10 N potassium
hydroxide to a pH of 10.5 ± 0.1.
BUFFER NO. 16:0.1 M, pH 7.0— Dissolve 13.6
g of dibasic potassium phosphate and 4.0 g of monobasic potassium phosphate in
1000 mL of water. Adjust with 18 N phosphoric acid or 10 N potassium hydroxide
to a pH of 7.0 ± 0.2.
UNITS AND REFERENCE STANDARDS:The potency of antibiotics is designated in either “Units” or “μg” of activity. In each case the “Unit” or “μg” of antibiotic
activity is established and defined by the designated federal master standard
for that antibiotic. The corresponding USP Reference Standard is calibrated in
terms of the master standard. USP Reference Standards for antibiotic substances
are held and distributed by the U.S. Pharmacopeial Convention, Inc.
PREPARATION OF THE STANDARDTo prepare a stock solution, dissolve a quantity of the
USP Reference Standard of a given antibiotic, accurately weighed, or the entire
contents of a vial of USP Reference Standard, where appropriate, in the solvent
specified in that table, and then dilute to the required concentration as
indicated. Store in a refrigerator, and use within the period indicated. On the
day of the assay, prepare from the stock solution five or more test dilutions,
the successive solutions increasing stepwise in concentration, usually in the ratio
of 1:1.25 for a cylinder-plate assay or smaller for a turbidimetric assay. Use
the final diluent specified and a sequence such that the middle or median has
the concentration designated.
PREPARATION OF THE SAMPLEFrom the information available for the preparation to be
assayed (the “Unknown”), assign to it
an assumed potency per unit weight or volume, and on this assumption prepare on
the day of the assay a stock solution and test dilution as specified for each
antibiotic but with the same final diluent as used for the USP Reference
Standard.
ORGANISMS AND INOCULUMTest OrganismsThe test organism for each antibiotic is listed in Table 2, together with its identification number in
the American Type Culture Collection. The method of assay is given for each in Table 1. Maintain a culture on slants of the medium
and under the incubation conditions specified in Table 3, and transfer weekly to fresh slants. For K. pneumonia use a noncapsulated culture. For Enterococcus hirae, stab cultures may be used.
Table 1. Preparation of Stock
Solutions and Test Dilutions of Reference Standards
NOTES—“B” denotes “buffer,” and the number
following refers to the potassium phosphate buffers defined
in this chapter.For amphotericin B, colistimethate sodium,
and nystatin, prepare
the USP Reference Standard solutions
and the sample test solution simultaneously.For amphotericin B, further dilute the stock solution with dimethyl sulfoxide
to give concentrations of 12.8, 16, 20, 25, and 31.2 µg per mL prior to making the test dilutions. The Test Dilution
of the sample
should contain the same amount
of dimethyl sulfoxide as the test dilutions of the USP Reference Standard.For bacitracin zinc,
each of the Standard test dilutions should
contain the same amount of hydrochloric acid as the Test Dilution
of the sample.For
neomycin turbidimetric assay, dilute
the 100-µg-per-mL stock
solution quantitatively with Buffer No. 3 to obtain
a solution having
a concentration equivalent to 25.0 µg of neomycin per mL. To separate
50-mL volumetric flasks
add 1.39, 1.67,
2.00, 2.40, and 2.88 mL of this solution, add 5.0 mL of 0.01 N hydrochloric acid to each flask, dilute with Buffer No. 3 to volume, and mix to obtain solutions
having concentrations of 0.69, 0.83, 1.0, 1.2, and 1.44 µg of neomycin per mL. Use these solutions
to prepare the standard response
line.For nystatin, further
dilute the stock solution with dimethylformamide to give concentrations of 256, 320, 400, 500, and 624 Units per mL prior to making the test dilutions. Prepare the standard
response line solutions
simultaneously with dilutions
of the sample to be tested. The Test Dilution of the sample should contain the same amount of dimethylformamide as the test dilutions of the Standard.
Use red low-actinic glassware.For Polymyxin B, prepare the stock solution
by adding 2 mL of water for each 5 mg of the weighed
USP Reference Standard
material.
Table 2. Test Organisms for
Antibiotics Assayed by the Procedure Indicated in Table 1
Antibiotic
|
Test Organism
|
ATCC * Number
|
Amikacin
|
Staphylococcus aureus
|
29737
|
Amphotericin B
|
Saccharomyces cerevisiae
|
9763
|
Bacitracin
|
Micrococcus luteus
|
10240
|
Bleomycin
|
Mycobacterium smegmatis
|
607
|
Candicidin
|
Saccharomyces cerevisiae
|
9763
|
Capreomycin
|
Klebsiella pneumonia
|
10031
|
Carbenicillin
|
Pseudomonas aeruginosa
|
25619
|
Cephalothin
|
Staphylococcus aureus
|
29737
|
Cephapirin
|
Staphylococcus aureus
|
29737
|
Chlorampheni- col
|
Escherichia coli
|
10536
|
Chlortetracy- cline
|
Staphylococcus aureus
|
29737
|
Cloxacillin
|
Staphylococcus aureus
|
29737
|
Colistimethate Sodium
|
Bordetella bronchiseptica
|
4617
|
Colistin
|
Bordetella bronchiseptica
|
4617
|
Cycloserine
|
Staphylococcus aureus
|
29737
|
Demeclocycline
|
Staphylococcus aureus
|
29737
|
Dihydrostrepto- mycin (CP)
|
Bacillus subtilis
|
6633
|
Dihydrostrepto- mycin (T)
|
Klebsiella pneumoniae
|
10031
|
Doxycycline
|
Staphylococcus aureus
|
29737
|
Erythromycin
|
Micrococcus luteus
|
9341
|
Gentamicin
|
Staphylococcus epidermidis
|
12228
|
Gramicidin
|
Enterococcus hirae
|
10541
|
Kanamycin
|
Staphylococcus aureus
|
29737
|
Methacycline
|
Staphylococcus aureus
|
29737
|
Nafcillin
|
Staphylococcus aureus
|
29737
|
Neomycin (CP)
|
Staphylococcus epidermidis
|
12228
|
Neomycin (T)
|
Klebsiella pneumoniae
|
10031
|
Netilmicin
|
Staphylococcus epidermidis
|
12228
|
Novobiocin
|
Staphylococcus epidermidis
|
12228
|
Nystatin
|
Saccharomyces cerevisiae
|
2601
|
Oxytetracycline
|
Staphylococcus aureus
|
29737
|
Paromomycin
|
Staphylococcus epidermidis
|
12228
|
Penicillin G
|
Staphylococcus aureus
|
29737
|
Polymyxin B
|
Bordetella bronchiseptica
|
4617
|
Rolitetracycline
|
Staphylococcus aureus
|
29737
|
Sisomicin
|
Staphylococcus epidermidis
|
12228
|
Spectinomycin
|
Escherichia coli
|
10536
|
Streptomycin (T)
|
Klebsiella pneumoniae
|
10031
|
Tetracycline
|
Staphylococcus aureus
|
29737
|
Thiostrepton (T)
|
Enterococcus hirae
|
10541
|
Tobramycin
|
Staphylococcus aureus
|
29737
|
Troleandomycin
|
Klebsiella pneumoniae
|
10031
|
Tylosin
|
Staphylococcus aureus
|
9144
|
Vancomycin
|
Bacillus subtilis
|
6633
|
Incubation Conditions
|
Suggested Inoculum Composition
|
|||||
Test Organism & (ATCC No.)
|
Medium
|
Temp. (
|
Time
|
Medium
|
Amount (mL per 100 mL)
|
Antibiotics Assayed
|
Bacillus subtilis
(6633)
|
32
|
32 to 35
|
5 days
|
5
|
As required
|
Dihydrostreptomycin
|
8
|
As required
|
Vancomycin
|
||||
Bordetella bronchiseptica
(4617)
|
1
|
32 to 35
|
24 hr.
|
10
|
0.1
|
Colistimethate Sodium, Colistin, Polymyxin B
|
Escherichia coli
|
1
|
32 to 35
|
24 hr.
|
3
|
0.7
|
Chloramphenicol
|
(10536)
|
||||||
Klebsiella pneumoniae
(10031)
|
1
|
36 to 37.5
|
16 to 24 hr.
|
3
|
0.05
|
Capreomycin
|
0.1
|
Streptomycin,
Troleandomycin, Dihydrostreptomycin
|
|||||
39
|
2
|
Neomycin
|
||||
Micrococcus luteus
|
1
|
32 to 35
|
24 hr.
|
11
|
1.5
|
Erythromycin
|
(9341)
|
||||||
Micrococcus luteus
(10240)
|
1
|
32 to 35
|
24 hr.
|
1
|
0.3
|
Bacitracin
|
Mycobacterium smegmatis (607)
|
36
|
36 to 37.5
|
48 hr.
|
35
|
1.0
|
Bleomycin
|
Pseudomonas aeruginosa
(25619)
|
1
|
36 to 37.5
|
24 hr.
|
10
|
0.5
|
Carbenicillin
|
Saccharomyces cerevisiae
(9763)
|
19
|
29 to 31
|
48 hr.
|
13
|
0.2
|
Candicidin
|
19
|
1.0
|
Amphotericin B
|
||||
Saccharomyces cerevisiae
(2601)
|
19
|
29 to 31
|
48 hr.
|
19
|
1.0
|
Nystatin
|
Staphylococcus aureus
(9144)
|
3
|
35 to 39
|
16 to 18 hr.
|
39
|
2-3
|
Tylosin
|
Staphylococcus aureus
|
1
|
32 to 35
|
24 hr.
|
1
|
0.1
|
Cephalothin, Cephapirin, Cloxacillin
|
(29737)
|
||||||
1
|
0.3
|
Nafcillin
|
||||
1
|
1.0
|
Penicillin G
|
||||
3
|
0.1
|
Amikacin, Chlortetracycline, Demeclocycline, Doxycycline, Methacycline, Oxytetracycline, Rolitetracycline,
Tetracycline
|
||||
3
|
0.2
|
Kanamycin
|
||||
3
|
0.4
|
Cycloserine
|
||||
3
|
0.15
|
Tobramycin
|
||||
Staphylococcus epidermidis
|
1
|
32 to 35
|
24 hr.
|
11
|
0.25
|
Netilmicin
|
(12228)
|
1
|
4.0
|
Novobiocin
|
|||
11
|
0.03
|
Gentamicin, Sisomicin
|
||||
11
|
0.4
|
Neomycin
|
||||
11
|
2.0
|
Paromomycin
|
||||
Enterococcus hirae
(10541)
|
3
|
36 to 37.5
|
16 to 18 hr.
|
3
|
1.0
|
Gramicidin
|
40
|
36 to 37.5
|
18 to 24 hr.
|
41
|
0.2
|
Thiostrepton
|
|
NOTE—For Pseudomonas aeruginosa (ATCC 25619)
in the assay
of Carbenicillin, use 0.5 mL of a 1:25 dilution of the stock
suspension per 100 mL of Medium
10.
|
||||||
Preparation of Inoculum
Preparatory to an assay, remove the growth from a recently grown slant or culture of the organism, with 3 mL of sterile saline TS and sterile glass beads. Inoculate the surface of 250 mL of the agar medium specified for that organism in Table 3 and contained on the flat side of a Roux bottle except in the case of Enterococcus hirae and Staphylococcus aureus (ATCC 9144), which are grown in a liquid medium. Spread the suspension evenly over the surface of the agar with the aid of sterile glass beads, and incubate at the temperature shown for approximately the indicated length of time. At the end of this period, prepare the stock suspension by collecting the surface growth in 50 mL of sterile saline TS, except for Bleomycin (use 50 mL of Medium 34).
Determine by trial the quantity of stock suspension to be used as the Inoculum, starting with the volume suggested in Table 3. The trial tests should be incubated for the times indicated in the section Turbidimetric Method for Procedure. Adjust the quantity of Inoculum on a daily basis, if necessary, to obtain the optimum dose-response relationship from the amount of growth of the test organism in the assay tubes and the length of the time of incubation. At the completion of the incubation periods described in the section Turbidimetric Method for Procedure, tubes containing the median dose of the Standard should have absorbances of at least 0.3 absorbance unit, except for Amikacin, Chlortetracycline, Gramicidin, and Tetracycline (0.35 absorbance unit), and Capreomycin, Methacycline, and Tobramycin (0.4 absorbance unit).
For the cylinder-plate assay, determine by trial the proportions of stock suspension to be incorporated in the Inoculum, starting with the volumes indicated in Table 3, that result in satisfactory demarcation of the zones of inhibition of about 14 to 16 mm in diameter and giving a reproducible dose relationship. Prepare the inoculum by adding a portion of stock suspension to a sufficient amount of agar medium that has been melted and cooled to 45 to 50 , and swirling to attain a homogeneous suspension.
PROCEDURE
Assay Designs
Microbial assays gain markedly in precision by the
segregation of relatively large sources of potential error and bias through
suitable experimental designs. In a cylinderplate assay, the essential
comparisons are restricted to relationships between zone diameter measurements
within plates, exclusive of the variation between plates in their preparation
and subsequent handling. To conduct a turbidimetric assay so that the
differences in observed turbidity will reflect the differences in the antibiotic
concentration requires both greater uniformity in the environment created for
the tubes through closer thermostatic control of the incubator and the
avoidance of systematic bias by use of a random placement of replicate tubes in
separate tube racks, each rack containing one complete set of treatments. The
essential comparisons are then restricted to relationships between the observed
turbidities within racks.
NOTE—For some purposes, the practice is to design
the assay so that a set of treatments consists of not fewer than three tubes
for each sample and standard concentration, and each set is placed in a single
rack.
Within these restrictions, the assay design recommended
is a 1-level assay with a standard curve. For this assay with a standard curve,
prepare solutions of 5, 6, or more test dilutions, provided they include one
corresponding to the reference concentration ( S 3), of the Standard and a solution of a
single median test level of the Unknown as described under Preparation of Standard and Preparation of the Sample. Consider an assay as preliminary if its
computed potency with either design is less than 80% or more than 125% of that
assumed in preparing the stock solution of the Unknown. In such a case, adjust
its assumed potency accordingly and repeat the assay.
Microbial determinations of potency are subject to
inter-assay as well as intra-assay variables, so that two or more independent
assays are required for a reliable estimate of the potency of a given assay
preparation or Unknown. Starting with separately prepared stock solutions and
test dilutions of both the Standard and the Unknown, repeat the assay of a
given Unknown on a different day. If the estimated potency of the second assay
differs significantly, as indicated by the calculated standard error, from that
of the first, conduct one or more additional assays. The combined result of a
series of smaller, independent assays spread over a number of days is a more
reliable estimate of potency than that from a single large assay with the same
total number of plates or tubes.
Cylinder-Plate Method
To prepare assay plates using Petri dishes, place 21 mL
of Medium 2 in each of the required number of plates, and allow it to harden into a
smooth base layer of uniform depth, except for Amphotericin B and Nystatin,
where no separate base layer is used. For Erythromycin, Gentamicin, Neomycin B,
Paromomycin, and Sisomicin, use Medium 11. For Bleomycin, use 10 mL of Medium 35. For Dihydrostreptomycin use Medium 5. For Vancomycin, use 10 mL of Medium 8. For Carbenicillin, Colistimethate Sodium,
Colistin, and Polymyxin B, use Medium 9. For Netilmicin, use 20 mL of Medium 11. Add 4 mL of seed layer inoculum (see Preparation of Inoculum and Table 3), prepared as directed for the given
antibiotic, except for Bleomycin (use 6 mL), for Netilmicin (use 5 mL), and for
Nystatin and Amphotericin B (use 8 mL), tilting the plate back and forth to
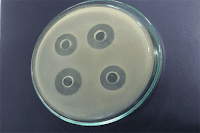
spread the inoculum evenly over the surface, and allow it to harden. Drop six
assay cylinders on the inoculated surface from a height of 12 mm, using a mechanical
guide or other device to insure even spacing on a radius of 2.8 cm, and cover
the plates to avoid contamination. After filling the six cylinders on each
plate with dilutions of antibiotic containing the test levels specified below, incubate
the plates at 32 to 35, or at the temperature specified below for the
individual case, for 16 to 18 hours, remove the cylinders, and measure and
record the diameter of each zone of growth inhibition to the nearest 0.1 mm.
Incubate the plates at 29 to 31 for Amphotericin B and Nystatin. Incubate at 34
to 36 for Novobiocin. Incubate at 36 to 37.5 for Carbenicillin, Colistimethate
Sodium, Colistin, Dihydrostreptomycin,
Turbidimetric Method
On the day of the assay, prepare the necessary doses by
dilution of stock solutions of the Standard and of each Unknown as defined
under Preparation of the Standard and Preparation of the Sample. Add 1.0 mL of each dose, except for
Gramicidin, Thiostrepton, and Tylosin (use 0.10 mL) to each of 3 prepared test
tubes, and place the 3 replicate tubes in a position, selected at random, in a
test tube rack or other carrier. Include similarly in each rack 1 or 2 control
tubes containing 1 mL of the test diluent (see Table 1) but no antibiotic. Upon completion of the
rack of test solutions (with Candicidin, within 30 minutes of the time when
water is added to the dimethyl sulfoxide stock solution), add 9.0 mL of
inoculum to each tube in the rack in turn, and place the completed rack
immediately in an incubator or a water bath maintained at 36 to 37.5, except
for Candicidin (incubate at 27 to 29). Incubate the tubes for 4 to 5 hours,
except for Capreomycin, Chloramphenicol, Cycloserine, Dihydrostreptomycin, Spectinomycin,
Streptomycin, and Troleandomycin (incubate these for 3 to 4 hours), Tylosin
(incubate for 3 to 5 hours), and Candicidin (incubate for 16 to 18 hours).
After incubation add 0.5 mL of dilute formaldehyde to each tube, except for
Tylosin (heat the rack in a water bath at 80 to 90 for 2 to 6 minutes or in a
steam bath for 5 to 10 minutes, and bring to room temperature), taking one rack
at a time, and read its transmittance or absorbance in a suitable spectrophotometer
fitted with a 530-nm or 580-nm filter (see Spectrophotometer under Apparatus).
CALCULATION
To calculate the potency from the data obtained either by
the cylinder-plate or by the turbidimetric method, using a log transformation,
straight-line method with a least-squares fitting procedure, and a test for linearity. Where a number of assays
of the same material are made with the same standard curve, calculate the
coefficient of variation of results of all of the assays of the material. Where more than one assay is
made of the same material with different standard curves, average the two or
more values of the potency.


Emoticon Emoticon